Molecular Neuroscience
Welcome to
The Molecular Neuroscience Section (Lab number 4)!
Department of Neurology,
Kyoto University Hospital and Graduate School of Medicine!
Alzheimer’s disease (AD) is characterized by the accumulation of senile plaques and hyperphosphorylated tau in the brain, leading to neurodegeneration and dementia. Only a limited number of therapies, which provide modest or no improvement of symptoms, are available for these pathological conditions. The goal of our research is to elucidate the causative mechanisms behind these disabling neurodegenerative diseases and discover novel therapies. |
||||
|
Figure 1. Hypothesis of our laboratory. |
||||
| KEY WORDS
Amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), Alzheimer’s disease (AD), SOD1, optineurin, Parkin, Pink1, DJ-1, ATP13A2, LRRK2, α-synuclein, amyloid precursor protein (APP), Presenilin1 (PS1), N-cadherin, mitochondria, misfolded proteins, ER stress, ubiquitin-proteasome system (UPS), induced pluripotent stem cell (iPS cell), high-throughput assay, medaka fish 1. Molecular pathogenesis and treatment of Amyotrophic Lateral Sclerosis (ALS) |
||||
| 1. Molecular pathogenesis and treatment of amyotrophic lateral sclerosis(ALS)
|
||||
|
||||
|
2. Parkinson’s disease (PD) Parkinson’s disease (PD) is the most common movement disorder, and the second most common neurodegenerative disorder after Alzheimer’s disease, affecting about 1-2% of people over 65 years. Although the etiology PD is still unknown, about 10% of PD cases are familial. Parkin, Pink1, DJ-1 and ATP13A2 are causative genes for autosomal recessive PD, while LRRK2 and α-synuclein are for autosomal dominant PD. (1) Autosomal recessive PD a) The role of Parkin and Pink1 especially on maintenance of mitochondrial function by using cellular systems and genetically engineered Drosophila and medaka fish.
b) Drug screening modifying functions and interactions of Parkin and Pink1.
Figure 3b. TILLING library of medaka fish
(2) Autosomal dominant PD a) The role of LRRK2 on cell signaling by using cellular system and genetically engineered Drosophila. |
||||
|
3. Molecular investigation of the pathogenesis of Alzheimer’s Disease (AD). AD is poised to become a significant public health crisis. The occurrence of AD is largely sporadic, typically affecting individuals over 65 years. Deposition of amyloid plaques is one of the pathological hallmarks of AD. Amyloid plaques are composed of β-amyloid (Aβ), which are derived from the amyloid precursor protein (APP) via proteolytic cleavage by β- and γ-secretases. Presenilin 1 (PS1), a causative gene for familial AD is found to be the enzymatic core of γ-secretase. A widely accepted hypothesis on AD pathogenesis is the amyloid hypothesis where Aβ plays a crucial role in neurodegeneration.
|
||||
| Current lab members: Ryosuke Takahashi, M.D., Ph.D.email: ryosuket¥kuhp.kyoto-u.ac.jp Hirofumi Yamashita, M.D., Ph.D.email: hirofumi¥kuhp.kyoto-u.ac.jp Hodaka Yamakado, M.D., Ph.D.email: yamakado¥kuhp.kyoto-u.ac.jp Akira Kuzuya, M.D., Ph.D.email: akuzuya¥kuhp.kyoto-u.ac.jp Norihito Uemura, M.D. Mayumi Akizuki, M.D., Ph.D. Yohsuke Taruno, M.D. (change ¥ to @ for email) Maiko Uemura, M.D. Miki Hishizawa, M.D. Masashi Ikuno, M.D. Kazuya Goto, M.D. Naoto Jingami, M.D. Hiroaki Masuda, M.D. Masakazu Miyamoto, M.D. Taro Okunomiya, M.D. Seiji Kaji, M.D. Etsuro Nakanishi, M.D. Hiroki Yagi, M.D. Shin-ya Okuda, M.D. Yasuhiro Fuseya, M.D. Masanori Sawamura, M.D. Tomonori Hoshino Tatsuhiro Ishikawa Rie Hikawa Megumi Asada Ryutaro Tamano Keiko Kimura Kumiko Imai Hitomi Nakabayashi Address correspondence: Department of Neurology, Kyoto University Graduate School of Medicine, 54 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto City, Kyoto 606-8507 Tel: +81-75-751-4397 Fax: +81-75-751-9780 |
| References
Parkin interacts with Klokin1 for mitochondrial import and maintenance of membrane potential. Environmental enrichment ameliorated high-fat diet-induced Aβ deposition and memory deficit in APP transgenic mice. Cell therapy for brain diseases: Perspective and future prospects. N-cadherin enhances APP dimerization at the extracellular domain and modulates Aβ production. P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. Presenilin regulates insulin signaling via a gamma-secretase-independent mechanism. Optineurin is co-localized with FUS in basophilic inclusions of ALS with FUS mutation and in basophilic inclusion body disease. Chemical library screening identifies a small molecule that downregulates SOD1 transcription for drugs to treat amyotrophic lateral sclerosis. The endoplasmic reticulum stress sensor, ATF6α, protects against neurotoxin-induced dopaminergic neuronal death. N-cadherin regulates p38 MAPK signaling via association with JNK-associated leucine zipper protein: implications for neurodegeneration in Alzheimer disease. Ammonium chloride and tunicamycin are novel toxins for dopaminergic neurons and induce Parkinson’s disease-like phenotypes in medaka fish. The loss of PGAM5 suppresses the mitochondrial degeneration caused by inactivation of PINK1 in Drosophila. Localization of HtrA2/Omi immunoreactivity in brains affected by Alzheimer’s disease. Proteasome inhibition in medaka brain induces the features of Parkinson’s disease. Insulin regulates Presenilin 1 localization via PI3K/Akt signaling. Induction of protective immunity by vaccination with wild-type apo superoxide dismutase 1 in mutant SOD1 transgenic mice. Mutations in LGI1 gene in Japanese families with autosomal dominant lateral temporal lobe epilepsy: the first report from Asian families. Loss of PINK1 in medaka fish (Oryzias latipes) causes late-onset decrease in spontaneous movement. PI3K inhibition causes the accumulation of ubiquitinated presenilin 1 without affecting the proteasome activity. [Pathogenesis of sporadic Parkinson’s disease: contribution of genetic and environmental risk factors]. A chemical neurotoxin, MPTP induces Parkinson’s disease like phenotype, movement disorders and persistent loss of dopamine neurons in medaka fish. 17th Symposium on the Treatment of Parkinson’s Disease. September 27, 2008, Tokyo. Foreword. [Frontier researches for the development of molecular-targeted therapies for familial Parkinson disease]. Edaravone in ALS. [Pathological mechanisms of Parkinson’s disease]. Nicotinic receptor stimulation protects nigral dopaminergic neurons in rotenone-induced Parkinson’s disease models. N-cadherin-based adhesion enhances Abeta release and decreases Abeta42/40 ratio. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. [Recent progress and future direction of neurodegenerative disease research]. Pael-R transgenic mice crossed with parkin deficient mice displayed progressive and selective catecholaminergic neuronal loss. Pael receptor is involved in dopamine metabolism in the nigrostriatal system. [Animal models for familial Parkinson’s disease]. Heat-shock protein 105 interacts with and suppresses aggregation of mutant Cu/Zn superoxide dismutase: clues to a possible strategy for treating ALS. [Pathogenesis of Parkinson disease]. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson’s disease. Cell type-specific upregulation of Parkin in response to ER stress. Presenilin 1 is involved in the maturation of beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1). [Ubiquitin and Parkinson’s disease]. |


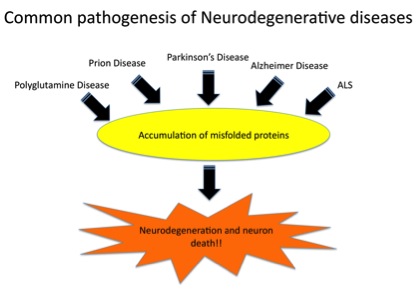
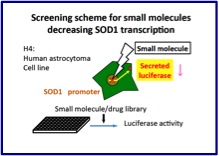 Figure 2a.
Figure 2a. Figure 2b.
Figure 2b.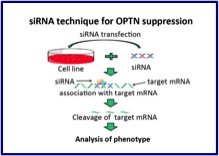 Figure 2c.
Figure 2c.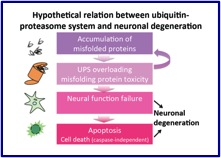 Figure 2d.
Figure 2d. Figure 3a. PINK1 and Parkin act together with Miro, to keep the mitochondrial quality control
Figure 3a. PINK1 and Parkin act together with Miro, to keep the mitochondrial quality control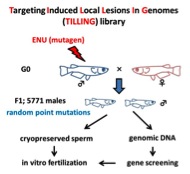

 a: olfactory bulb,
a: olfactory bulb,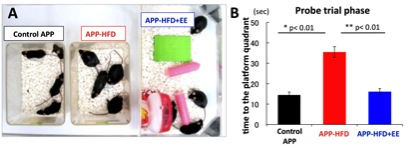 Figure 4. A: APP-high fat diet (HFD) model (mimicking metabolic syndrome) and environmental enrichment (EE). B: HFD worsens the cognitive function of APP model mice, whereas EE ameliorates it (demonstrated by Morris Water Maze test).
Figure 4. A: APP-high fat diet (HFD) model (mimicking metabolic syndrome) and environmental enrichment (EE). B: HFD worsens the cognitive function of APP model mice, whereas EE ameliorates it (demonstrated by Morris Water Maze test).