Neuropathology

Welcome to
The Section of Neuropathology (Lab number 1st/2nd)!,
Department of Neurology,
Kyoto University Hospital and Graduate School of Medicine!
|
Our lab was first established in 1983 under the appointment of Prof. Masakuni KAMEYAMA who specialized in neuropathology of cerebrovascular disorder. Since the opening, we consistently continue our research on cerebrovascular, neurodegenerative and neuroinflammatory diseases, to improve our understanding of how disease starts and progresses and to help develop new treatments. Neuropathological research is mainly grouped into two methodologies; human neuropathology investigating autopsied brain and experimental neuropathology examining disease-model animal. Recently, we also apply rapidly evolving in vivo imaging techniques such as MRI and PET to visualize pathological process in the living brain. With these methodologies, the aim is to elucidate morphological changes directly connected to the disease and to lead to cures. We explore neuropathological tradition to the full and innovate new vision and technique in order for the success of the projects below. Human neuropathology investigating autopsied brain: Our lab holds an extensive archive of tissue samples donated by individuals with neurological disease. The donated samples from the brain (376 in total) and other organs of the body were collected during post mortem examination at our Neurology department and affiliated hospitals. Under closely controlled conditions, tissue is stored as formalin fixed, paraffin embedded blocks and is frozen. The archive could not provide support for medical research, were it not for the generosity of patients and families who are able to donate tissue samples and organs despite their personal loss and grief. |
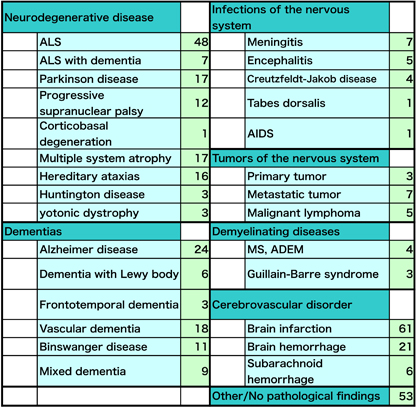
| Utilizing the donated samples, we are investigating the biological process underlying amyotrophic lateral sclerosis (ALS), Parkinson disease and movement disorders, vascular dementia and Alzheimer disease. The tissue samples are essential to validate the hypotheses and observations generated by the cell biology and biochemical studies of disease-model animals. |
|
Experimental neuropathology examining disease-model animal: Ongoing research on neurodegenerative disorders aims at identifying essential pathological mechanisms in ALS and Parkinson disease. To achieve the aim, we employ multidisciplinary approach involving molecular genetics, neurochemical and neurophysiological analyses. In addition to the Molecular Neuroscience Section (Lab number 4) of our Neurology department, we collaborate with a number of research institutions that are listed below: 1) Department of Epidemiology, Division of Radiation Bio-Medical Informatics, Research Institute for Radiation Biology and Medicine, Hiroshima University, Professor Hideshi Kawakami 2) Center for iPS Cell Research and Application, Kyoto University, Associate Professor Haruhisa Inoue 3) Center for iPS Cell Research and Application, Kyoto University, Professor Jun Takahashi 4) Unit for Neurobiology and Therapeutics, Molecular Neuroscience Research Center, Shiga University of Medical Science, Associate Professor Makoto Urushitani 5) Department of Neurology, Tokushima University Graduate School of Medicine, Professor Ryuji Kaji 6) Department of Neurology, Tohoku University School of Medicine, Professor Masashi Aoki 7) Montefiore Medical Center, Albert Einstein College of Medicine, Professor Asao Hirano In the last five years, studies have identified multiple mechanisms by which gene mutations related to ALS cause the disease. These discoveries open a new field for ALS research. Analysis of the genes’ protein products and their role in cell death may provide a clue to develop new treatments and find a cure for the disease. |
|
In vivo imaging research: Our research aims at increasing our understanding of the mechanisms and causes leading to brain degeneration and dysfunction in Parkinson disease, Dementia with Lewy body, vascular dementia and Alzheimer disease. Our mission is to transform clinical problems into testable research hypotheses and to translate research findings into medical advances using state-of-the-art in vivo imaging techniques. Recent advances in in vivo imaging techniques allowed the use of these methodologies to visualize pathological findings in the living individual, which were previously identified only after death by an autopsied tissue sample. For example, a PET technique with Pittsburgh compound B (PIB) now enables us to visualize deposits of beta-amyloid, one pathological hallmark of Alzheimer’s disease, in the living brain, which were previously identified only in an autopsied brain or a tissue sample of disease-model animal. The discovery of PIB PET technique not only expands our knowledge about Alzheimer disease but also opens the new possibility of pre-symptomatic intervention. We believe that development of tau and alpha-synuclein in vivo imaging agents makes a dramatic progress to improve our understanding of how neurodegenerative disorders start and progress. |
|
Research on neuroinflammatory diseases: The aims of our research are to clarify biological process underlying multiple sclerosis and neuromyelitis optica (NMO). We collaborate with number of research institutions including Kyoto Prefectural University of Medicine, National Hospital Organization Utano Hospital, Koseikai Takeda Hospital and Nagasaki University. |
|
Current lab members:
Address correspondence: |
|
Publications in 2012 and 2011 【2012】 1. Kubota M, Miyata J, Sasamoto A, Sugihara G, Yoshida H, Kawada R, Fujimoto S, Tanaka Y, Sawamoto N, Fukuyama H, Takahashi H, Murai T: Thalamocortical Disconnection in the Orbitofrontal Region Associated With Cortical Thinning in Schizophrenia. Arch Gen Psychiatry, in press. 2. Okuchi S, Okada T, Ihara M, Gotoh K, Kido A, Fujimoto K, Yamamoto A, Kanagaki M, Tanaka S, Takahashi R, Togashi K: Visualization of Lenticulostriate Arteries by Flow-Sensitive Black-Blood MR Angiography on a 1.5T MRI System: A Comparative Study between Subjects with and without Stroke. AJNR Am J Neuroradiol, in press. 3. Ihara M, Okamoto Y, Takahashi R: Suitability of the Montreal Cognitive Assessment versus the Mini-Mental State Examination in Detecting Vascular Cognitive Impairment. J Stroke Cerebrovasc Dis, in press. 4. Ihara M, Okamoto Y, Hase Y, Takahashi R: Association of Physical Activity with the Visuospatial/Executive Functions of the Montreal Cognitive Assessment in Patients with Vascular Cognitive Impairment. J Stroke Cerebrovasc Dis, in press. 5. Kubota M, Miyata J, Sasamoto A, Kawada R, Fujimoto S, Tanaka Y, Sawamoto N, Fukuyama H, Takahashi H, Murai T: Alexithymia and reduced white matter integrity in schizophrenia: a diffusion tensor imaging study on impaired emotional self-awareness. Schizophr Res, 141: 137-43, 2012. 6. Mihara M, Hara H, Hayashi Y, Narushima M, Yamamoto T, Todokoro T, Iida T, Sawamoto N, Araki J, Kikuchi K, Murai N, Okitsu T, Kisu I, Koshima I: Pathological steps of cancer-related lymphedema: histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One, 7: e41126, 2012. 7. Aotani D, Ebihara K, Sawamoto N, Kusakabe T, Aizawa-Abe M, Kataoka S, Sakai T, Iogawa H, Ebihara C, Fujikura J, Hosoda K, Fukuyama H, Nakao K: Functional magnetic resonance imaging analysis of food-related brain activity in patients with lipodystrophy undergoing leptin replacement therapy. J Clin Endocrinol Metab, 97: 3663-71, 2012. 8. Matsuyoshi D, Ikeda T, Sawamoto N, Kakigi R, Fukuyama H, Osaka N: Differential roles for parietal and occipital cortices in visual working memory. PLoS One, 7: e38623, 2012. 9. Inoue M, Kojima Y, Mima T, Sawamoto N, Matsuhashi M, Fumuro T, Kinboshi M, Koganemaru S, Kanda M, Shibasaki H: Pathophysiology of unilateral asterixis due to thalamic lesion. Clin Neurophysiol, 123: 1858-64, 2012. 10. Otsuka Y, Yamauchi H, Sawamoto N, Iseki K, Tomimoto H, Fukuyama H: Diffuse tract damage in the hemispheric deep white matter may correlate with global cognitive impairment and callosal atrophy in patients with extensive leukoaraiosis. AJNR Am J Neuroradiol, 33: 726-32, 2012. 11. Miyata J, Sasamoto A, Koelkebeck K, Hirao K, Ueda K, Kawada R, Fujimoto S, Tanaka Y, Kubota M, Fukuyama H, Sawamoto N, Takahashi H, Murai T: Abnormal asymmetry of white matter integrity in schizophrenia revealed by voxelwise diffusion tensor imaging. Hum Brain Mapp, 33: 1741-9, 2012. 12. Tashiro Y, Urushitani M, Inoue H, Koike M, Uchiyama Y, Komatsu M, Tanaka K, Yamazaki M, Abe M, Misawa H, Sakimura K, Ito H, Takahashi R: Motor Neuron-specific Disruption of Proteasomes, but Not Autophagy, Replicates Amyotrophic Lateral Sclerosis. J Biol Chem, 287: 42984-94, 2012. 13. Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, Yamamoto T, Adachi F, Kondo T, Okita K, Asaka I, Aoi T, Watanabe A, Yamada Y, Morizane A, Takahashi J, Ayaki T, Ito H, Yoshikawa K, Yamawaki S, Suzuki S, Watanabe D, Hioki H, Kaneko T, Makioka K, Okamoto K, Takuma H, Tamaoka A, Hasegawa K, Nonaka T, Hasegawa M, Kawata A, Yoshida M, Nakahata T, Takahashi R, Marchetto MC, Gage FH, Yamanaka S, Inoue H: Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med, 4: 145ra104, 2012. 14. Honjo Y, Ito H, Horibe T, Shimada H, Nakanishi A, Mori H, Takahashi R, Kawakami K: Derlin-1-immunopositive inclusions in patients with Alzheimer’s disease. Neuroreport, 23: 611-5, 2012. 15. Kawamoto Y, Ito H, Ihara M, Takahashi R: Immunohistochemical localization of X-linked inhibitor of apoptosis protein in brainstem-type and cortical Lewy bodies. Neuroreport, 23: 162-7, 2012. 16. Kasahara S, Miki Y, Kanagaki M, Kondo T, Yamamoto A, Morimoto E, Okada T, Ito H, Takahashi R, Togashi K. “Hot cross bun” sign in multiple system atrophy with predominant cerebellar ataxia: a comparison between proton density-weighted imaging and T2-weighted imaging. Eur J Radiol, 81: 2848-52, 2012. 17. Hase Y, Okamoto Y, Fujita Y, Kitamura A, Nakabayashi H, Ito H, Maki T, Washida K, Takahashi R, Ihara M. Cilostazol, a phosphodiesterase inhibitor, prevents no-reflow and hemorrhage in mice with focal cerebral ischemia. Exp Neurol, 233: 523-33, 2012. 18. Okamoto Y, Yamamoto T, Kalaria RN, Senzaki H, Maki T, Hase Y, Kitamura A, Washida K, Yamada M, Ito H, Tomimoto H, Takahashi R, Ihara M. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol, 123: 381-94. 2012. 19. Kitamura A, Fujita Y, Oishi N, Kalaria RN, Washida K, Maki T, Okamoto Y, Hase Y, Yamada M, Takahashi J, Ito H, Tomimoto H, Fukuyama H, Takahashi R, Ihara M: Selective white matter abnormalities in a novel rat model of vascular dementia. Neurobiol Aging, 33: 1012.e25-35, 2012. 20. Fujita Y, Kuchimaru T, Kadonosono T, Tanaka S, Hase Y, Tomimoto H, Hiraoka M, Kizaka-Kondoh S, Ihara M, Takahashi R: In vivo imaging of brain ischemia using an oxygen-dependent degradative fusion protein probe. PLoS One, 7: e48051, 2012. 21. Watanabe H, Ono M, Kimura H, Matsumura K, Yoshimura M, Okamoto Y, Ihara M, Takahashi R, Saji H: Synthesis and biological evaluation of novel oxindole derivatives for imaging neurofibrillary tangles in Alzheimer’s disease. Bioorg Med Chem Lett, 22: 5700-3, 2012. 22. Cui M, Ono M, Kimura H, Ueda M, Nakamoto Y, Togashi K, Okamoto Y, Ihara M, Takahashi R, Liu B, Saji H: Novel (18)F-Labeled Benzoxazole Derivatives as Potential Positron Emission Tomography Probes for Imaging of Cerebral β-Amyloid Plaques in Alzheimer’s Disease. J Med Chem, 55: 9136-45, 2012. 23. Shimazu Y, Minakawa EN, Nishikori M, Ihara M, Hashi Y, Matsuyama H, Hishizawa M, Yoshida S, Kitano T, Kondo T, Ishikawa T, Takahashi R, Takaori-Kondo A. A case of follicular lymphoma associated with paraneoplastic cerebellar degeneration. Intern Med, 51: 1387-92, 2012. 24. Maesako M, Uemura K, Kubota M, Kuzuya A, Sasaki K, Asada M, Watanabe K, Hayashida N, Ihara M, Ito H, Shimohama S, Kihara T, Kinoshita A: Environmental enrichment ameliorated high fat diet-induced Aβ deposition and memory deficit in APP transgenic mice. Neurobiol Aging, 33: 1011.e11-23, 2012. 25. Komori M, Matsuyama Y, Nirasawa T, Thiele H, Becker M, Alexandrov T, Saida T, Tanaka M, Matsuo H, TomimotoH, Takahashi R, Tashiro K, Ikegawa M, Kondo T. Proteomic pattern analysis discriminates among multiple sclerosis-related disorders. Ann Neurol, 71: 614-23, 2012 |
|
【2011】 1. Maki T, Ihara M, Fujita Y, Nambu T, Miyashita K, Yamada M, Washida K, Nishio K, Ito H, Harada H, Yokoi H, Arai H, Itoh H, Nakao K, Takahashi R, Tomimoto H: Angiogenic and vasoprotective effects of adrenomedulin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke, 42: 1122-1128, 2011. 2. Yamada M, Ihara M, Okamoto Y, Maki T, Washida K, Kitamura A, Hase Y, Ito H, Takao K, Miyakawa T, Kalaria RN, Tomimoto H, Takahashi R: The influence of chronic cerebral hypoperfusion on cognitive function and amyloid b metabolism in APP overexpressing mice. PLoS One, 6: e16567, 2011. 3. Ito H, Fujita K, Nakamura M, Wate R, Kaneko S, Sasaki S, Yamane K, Suzuki N, Aoki M, Shibata N, Togashi S, Kawata A, Mochizuki Y, Mizutani T, Maruyama H, Hirano A, Takahashi R, Kawakami H, Kusaka H: Optineurin is co-localized with FUS in basophilic inclusions of ALS with FUS mutation and in basophilic inclusion body disease. Acta Neuropathol, 121: 555-557, 2011. 4. Maki T, Ihara M, Fujita Y, Nambu T, Harada H, Ito H, Nakao K, Tomimoto H, Takahashi R: Angiogenic roles of adrenomedullin through VEGF induction. Neuroreport, 22: 442-447, 2011. 5. Okamoto Y, Shirakashi Y, Ihara M, Urushitani M, Oono M, Kawamoto Y, Yamashita H, Shimohama S, Kato S, Hirano A, Tomimoto H, Ito H, Takahashi R: Colocalization of 14-3-3 proteins with SOD1 in Lewy Body-like hyaline inclusions in familial amyotrophic lateral sclerosis cases and the animal model. PLoS One, 6: e20427, 2011. 6. Honjo Y, Ito H, Horibe T, Takahashi R, Kawakami K: Protein disulfide isomerase immunopositive glial cytoplasmic inclusions in patients with multiple system atrophy. Int J Neurosci, 121: 543-50, 2011. 7. Maki T, Wakita H, Mase M, Itagaki I, Saito N, Ono F, Adachi K, Ito H, Takahashi R, Ihara M, Tomimoto H: Watershed Infarcts in a multiple microembolic model of monkey. Neurosci Lett, 499: 80-83, 2011. 8. Ito H, Nakamura M, Komure O, Ayaki T, Wate R, Maruyama H, Nakamura Y, Fujita K, Kaneko S, Okamoto Y, Ihara M, Konishi T, Ogasawara K, Hirano A, Kusaka H, Kaji R, Takahashi R, Kawakami H: Clinicopathologic study on an ALS family with a heterozygous E478G optineurin mutation. Acta Neuropathol, 122: 223-229, 2011. 9. Honjo Y, Kaneko S, Ito H, Horibe T, Takahashi R, Kawakami K: Protein disulfide isomerase immunopositive inclusions in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler, 12: 444-450, 2011. 10. Saito S, Ozaki A, Takahashi M, Ito H, Matsumoto S, Tomimoto H: Clustering of multifocal cerebral infarctions in CADASIL: a case report. J Neurol, 258: 325-327, 2011. 11. Matsuya N, Komori M, Nomura K, Nakane S, Fukudome T, Goto H, Shiraishi H, Wandinger KP, Matsuo H, Kondo T. Increased T-cell immunity against aquaporin-4 and proteolipid protein in neuromyelitis optica. Int Immunol, 23: 565-573, 2011. 12. Okamoto Y, Ihara M, Urushitani M, Yamashita H, Kondo T, Tanigaki A, Oono M, Kawamata J, Ikemoto A, Kawamoto Y, Takahashi R, Ito H: An autopsy case of SOD1-related ALS with TDP-43-positive inclusions. Neurology, 77:1993-1995, 2011. |

